![The crystal structure of trans potassium dioxalatodiaquochromiate, K[CR(C2O4)2(H2O)2].3H2O - Van Niekerk - 1951 - Acta Crystallographica - Wiley Online Library The crystal structure of trans potassium dioxalatodiaquochromiate, K[CR(C2O4)2(H2O)2].3H2O - Van Niekerk - 1951 - Acta Crystallographica - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/29dbd8f8-5ed2-49f2-94d2-4332b7d55fcc/s0365110x51000064.fp.png)
The crystal structure of trans potassium dioxalatodiaquochromiate, K[CR(C2O4)2(H2O)2].3H2O - Van Niekerk - 1951 - Acta Crystallographica - Wiley Online Library

Overall reaction of Cathode and Anode: Cathode: 2 H2O(l) + 2e− → H2(g)... | Download Scientific Diagram

Synthesis of cis-Cu(gly)2·H2O, trans-Cu(gly)2, and cis-Ni(gly)2(H2O)2 and their Characterization Using Thermal and Spectroscopic Techniques—A Capstone Inorganic Laboratory | Journal of Chemical Education

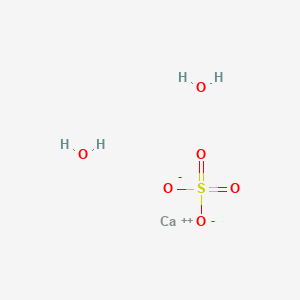
Complete the following equation: Na2O2 + 2H2O → (W) + H2O2 2KO2 + 2H2O→ (X) + (Y) + O2 Na2O + CO2→ (Z)
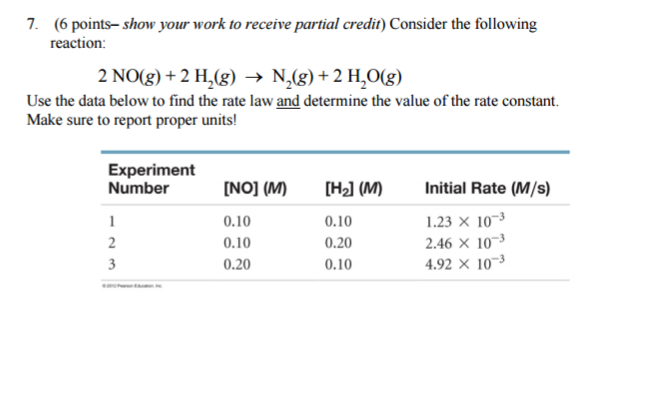
Consider the following reaction: 2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g) Use the data below to find the rate law and determine the value of the rate constant? Make